On March 11, 2024, the official website of the State Food and Drug Administration showed that the atropine-sulfate eye drops (SQ-729) of Xingqi Ophthalmic Medicine was approved for listing, which is used to delay the progression of myopia in children aged 6 to 12 years with a spheroscope degree of -1.00D to -4.00D (astigmatism ≤1.50D, anisometropia ≤1.50D). It became the first domestic market low-concentration atropine sulfate eye drops to delay the progression of myopia in children.
The current forecast peak sales may reach 10.1 billion yuan. At present, the prevalence of myopia in the world is increasing rapidly. It is estimated that by 2050, there will be 4.758 billion people with myopia in the world, accounting for 49.8% of the total population. In China, data from the National Health Commission show that the prevalence of myopia among children and adolescents in China in 2020 will be 52.7%, including 35.6% for primary school students, 71.1% for middle school students and 80.5% for high school students. According to CICC estimates, by 2030, China's myopia prevention and control market scale or 210 billion yuan, ten years compound growth rate of about 13.7%.
In the past, the ophthalmology market related products are mainly equipment consumables, including artificial lenses, orthokeratology lenses (OK glasses), rigid contact lenses (contact lenses), etc. For pharmaceutical companies, the approval of new products means that they can get a share of the huge market. According to Frost and Sullivan, the market size of ophthalmology treatment drugs will reach 44 billion yuan by 2025 and 108.4 billion yuan by 2030, a market that will double in five years, which is very attractive.
Looking at the overall ophthalmic circuit, the ophthalmic service market accounts for the largest proportion, reaching about 71%; Equipment followed, accounting for about 17%; Ophthalmic drugs currently account for the smallest proportion of the market, while developing the most rapidly, the growth rate around 2021 is about 15%. As the number of patients increases and the penetration rate of ophthalmic drugs increases, its market size will continue to expand.
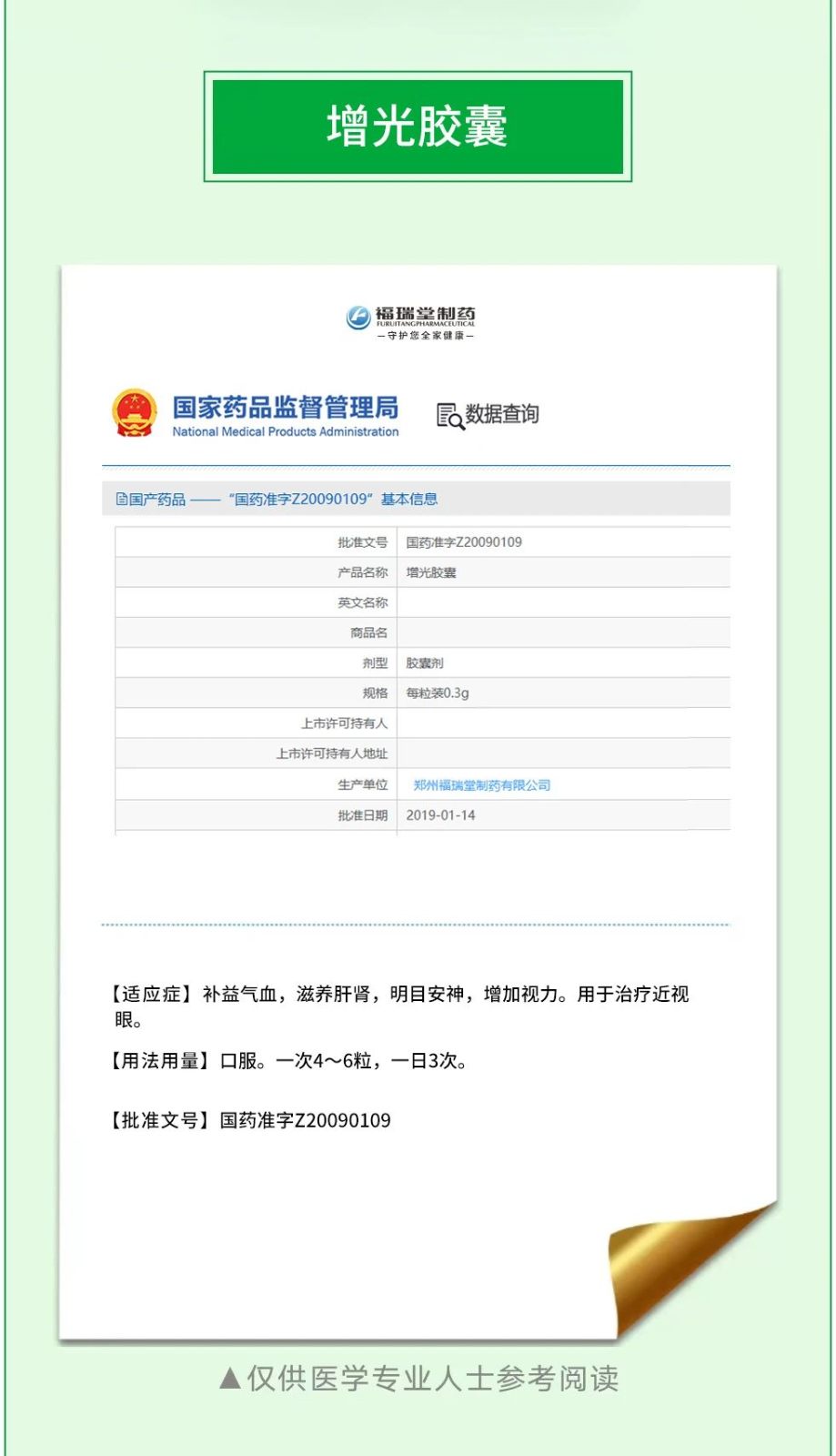
Furuitang Pharmaceutical as a high-tech enterprise in Zhengzhou City, one of the new Chinese medicine treatment myopia products - Furuitang Zengguang capsule in 2009 was approved by the State Food and Drug Administration, the national drug approval number Z20090109, Zengguang capsule by Codonopsis, angelica, goji berry, poria, ophiopogon, alissia, schisandra, calamus, peony bark, polygala (licorice water) and other 10 flavors of Chinese medicinal materials carefully processed, the State Food and Drug Administration clearly approved the function of: tonifying Qi and blood, nourishing the liver and kidney, brightening the eyes and calming the mind, increase vision. It is used to treat myopia.
"Electronic Journal of Clinical Medical Literature", volume 6, issue 71, 2019, led by Sun Jun of Lixian Hospital of Traditional Chinese Medicine, Changde City, Hunan Province, the clinical therapeutic effect of Zengguang capsule in patients with myopia and its impact on drug safety and vision, the article concluded that Zengguang capsule has been applied in patients with myopia, and the effect is ideal. Zengguang capsule is a Chinese drug. In this study, there were no statistically significant differences in naked eye visual acuity, diopter and axial length between the two groups before treatment (p> 0.05) : The naked eye visual acuity and axial length of eye in observation group after treatment were higher than those in control group (p< 0.05); The diopter of the observation group at 1 year after treatment was lower than that of the control group (p< 0.05), indicating that Zengguang capsule can obtain good therapeutic effect in patients with myopia, which is helpful to improve patients' vision and reduce diopter, which is conducive to patients' rehabilitation. Zengguang capsules are commonly used in the treatment of myopia patients. The formula is composed of Radix angelicae, alisma, Angelica, Wolfberry, poria, ophiopogon, calamus, Moutan bark, schisandra and Radix polygala (made of liquorice water). Since Zengguang capsule is a Chinese drug, the drug safety is relatively high in clinical use, which helps to improve patient tolerance and compliance. In this study, there was no statistical significance in the incidence of dry eyes, elevated intracranial pressure, abnormal blood pressure, dizziness, tinnitus and drug allergy in the two groups during treatment (P > 0.05), indicating that Zengguang capsule has higher drug safety and lower incidence of drug complications in patients with myopia. However, due to the different causes of each myopia patient, the treatment process should strengthen the treatment of patients with basic diseases, dynamic monitoring of patients' vision, timely adjustment of treatment plans, and promote early recovery of patients.